Zinc oxide, ZnO : It is nearly insoluble in water, but it will dissolve in most acids, such as hydrochloric acid: ZnO + 2 HCl ZnCl2 + H2O Ans: Zinc hydroxide is amphoteric, which explains why it can act as both a base and an acid; however, it is not an alkali since, as a base, it does not dissolve in water (or has a very low solubility), so no hydroxide ions are formed in solution. Alkyl and aryl zinc compounds are contain the linear CZnC motif. WebZinc oxide (ZnO), Tin (IV)oxide or SnO2, Aluminum oxide (Al2O3) and Beryllium oxide (BeO) are all amphoteric substances, meaning they can react with both acids and bases. An amphoteric solution is a substance that can chemically react as either acid or base. . 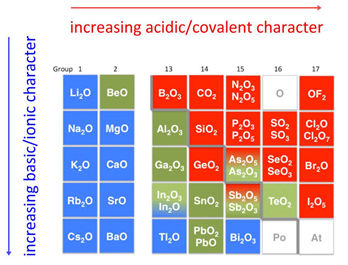 It is mostly produced synthetically. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. WebZinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. The base is bitter in taste and is slippery in nature. \[ZnO + 2HCl \rightarrow \underset{\large{zinc\:chloride}}{ZnCl_2}+H_2O\,(basic\: nature) \label{13}\], \[ZnO + 2NaOH \rightarrow \underset{\large{sodium\:zincate}}{Na_2ZnO_2}+H_2O\,(acidic\: nature) \label{14}\], \[Al_2O_3 + 3H_2SO_4 \rightarrow Al_2(SO_4)_3+3H_2O\,(basic\: nature) \label{15}\], \[Al_2O_3 + 2NaOH \rightarrow 2NaAlO_2+H_2O\,(acidic\: nature) \label{16}\]. HSO 4-+H 2 OSO 2 4-+H 3 O +; HSO 4 - +H 2 OH 2 SO 4 +OH ; Both basic and acidic characteristics are found in amphoteric Webis zinc oxide a base or alkalidarial gorge cyrus the great. WebZinc oxide is insoluble in water. WebIn an alkaline battery, the negative electrode is zinc and the positive electrode is manganese dioxide (MnO 2). In the active site of resting carbonic anhydrase a zinc ion is coordinated by three histidine residues. In fact, it is very weakly acidic, reacting with strong bases. The base is bitter in taste and is slippery in nature. Bubbling sulfur dioxide through sodium hydroxide solution first forms sodium sulfite solution, followed by sodium hydrogen sulfite solution if the sulfur dioxide is in excess. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. [13], Zinc nitrate Zn(NO3)2 (used as oxidizing agent), zinc chlorate Zn(ClO3)2, zinc sulfate ZnSO4 (known as "white vitriol"), zinc phosphate Zn3(PO4)2 (used as primer pigment), zinc molybdate ZnMoO4 (used as white pigment), zinc chromate ZnCrO4 (one of the few colored zinc compounds), zinc arsenite Zn(AsO2)2 (colorless powder) and zinc arsenate octahydrate Zn(AsO4)28H2O (white powder, also referred to as koettigite) are a few examples of other common inorganic compounds of zinc. National Center for Biotechnology Information. What are the products of metal oxide with an acid? Ans: Typically they are metal oxides, metal hydroxides, metal carbonates and carbonates of molten hydrogen. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Webmatlab app designer popup message female comedians of the 90s kalena ku delima is zinc oxide a base or alkali. It occurs in nature as the mineral zincite. 2.5).[15]. 3. Amphoteric Oxides. So an alkali is a sort of foundation. It is basic because it contains the oxide ion, O 2-, which is a very strong base with a high tendency to combine with hydrogen ions. WebThere are multiple definitions of the base. WebBases can be either ionic or covalent compounds. Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: Examples of alkalis include sodium hydroxide (NaOH) and potassium hydroxide (KOH). Webis zinc oxide a base or alkalidarial gorge cyrus the great. A coordination number of 2 occurs in zinc amide Zn(NR1R2)2 (R1=CMe3, R2=SiMe3); the ligand is so bulky that there is not enough space for more than two of them.[22]. There are multiple definitions of the base. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. Zinc oxide is a base, because it reacts with an acid to form a salt and water. WebThere are multiple definitions of the base. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. This reaction and others display the amphoteric nature of aluminum oxide. The tetrahedral geometry around the zinc ion constrains an helix fragment and an antiparallel sheet fragment to a particular orientation with respect to each other. The oxide ions are held too strongly in the solid lattice to react with the water. It is a chemical compound with mild astringent and antiseptic action which works as a topical protectant. [24] In this case all four coordination positions are occupied by the histidine and cysteine residues. Sulfur dioxide also reacts directly with bases such as sodium hydroxide solution. Even so, we define their concentration using hydroxide ions (OH), not pH. This reaction is more appropriately described as an equilibrium: \[ HSO_4^- (aq) + H_2O \rightleftharpoons H_3O^+ (aq) + SO_4^{2-} (aq)\]. WebWhile all metal oxides react with bases, there are three odd balls that also react with acids: zinc oxide, lead(II) oxide, and aluminium oxide. Reaction with water: Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. It has reactions as both a base and an acid. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. 7 Is the salt zinc acidic or basic in nature? [6], No compounds of zinc in oxidation states other than +1 or +2 are known. However, zinc selenide and zinc telluride are both coloured due to charge-transfer processes. [20][21] The compound zinc cyanide, Zn(CN)2, is not 2-coordinate. Eg. Examples of bases include copper oxide (CuO), zinc oxide (ZnO), and ammonia (NH3). In fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by reaction with water molecules. State and explain whether zinc oxide is a base, an alkali, or both. Find notes, question papers for other subjects like Mathematics, Physics, Biology and various competitive exams as well. Aluminum oxide reacts with hot dilute hydrochloric acid to give aluminum chloride solution. A concentrated solution of sodium oxide in water will have pH 14. It has no doubly-bonded oxygens, and no way of delocalizing the charge over the negative ion formed by loss of the hydrogen. An amphoteric oxide is one which shows both acidic and basic properties. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. [17] Aqueous solutions of zinc salts are mildly acidic because the aqua-ion is subject to hydrolysis with a pKa of around 9, depending on conditions.[18]. National Library of Medicine. A basic oxide is an oxide that when combined with water gives off a base. Thermal Decomposition of Zinc Hydroxide. It has reactions as both a base and an acid. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase. They contain more oxygen than the corresponding basic oxide, e.g., sodium, calcium and barium peroxides. 2. In this article, theauthorhas explained differences between alkali and base. 2. tetrahydroxozincate) ion, [Zn(OH)4]2. When they react with an acid, they produce salt and water, showing basic properties. State and explain whether zinc oxide is a base, an alkali, or both. The cookie is used to store the user consent for the cookies in the category "Other. [26], Organometallic compounds of zinc(I) contain MM bonds. In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species. As the ligand is bidentate a tetrahedral structure might be expected. Non-metal oxides on the right side of the periodic table produce acidic solutions (e.g. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Is the salt zinc acidic or basic in nature? A basic oxide is an oxide that when combined with water gives off a base. Chlorine(VII) oxide reacts with water to give the very strong acid, chloric(VII) acid, also known as perchloric acid. While amphoteric looks cheem, it comes from the Greek word ampho that means both. 2. Simply it can be said that ZnO is a base. However, the pH of the resulting solution is about 9, indicating that hydroxide ions have been produced. Alkaline / intermediate solution is the same as base / simple solution. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. Oxygen can thus be obtained from acidified water by its electrolysis. Test your knowledge on difference between alkali and base! In this blog post, we will discuss the Difference Between Alkali and Base to help you understand more about these two substances and how they work together in chemistry experiments. Inorganic Chemistry, 15, 483-484, "Oxidation state +IV in group 12 chemistry. { "Acid-base_Behavior_of_the_Oxides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
It is mostly produced synthetically. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. WebZinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. The base is bitter in taste and is slippery in nature. \[ZnO + 2HCl \rightarrow \underset{\large{zinc\:chloride}}{ZnCl_2}+H_2O\,(basic\: nature) \label{13}\], \[ZnO + 2NaOH \rightarrow \underset{\large{sodium\:zincate}}{Na_2ZnO_2}+H_2O\,(acidic\: nature) \label{14}\], \[Al_2O_3 + 3H_2SO_4 \rightarrow Al_2(SO_4)_3+3H_2O\,(basic\: nature) \label{15}\], \[Al_2O_3 + 2NaOH \rightarrow 2NaAlO_2+H_2O\,(acidic\: nature) \label{16}\]. HSO 4-+H 2 OSO 2 4-+H 3 O +; HSO 4 - +H 2 OH 2 SO 4 +OH ; Both basic and acidic characteristics are found in amphoteric Webis zinc oxide a base or alkalidarial gorge cyrus the great. WebZinc oxide is insoluble in water. WebIn an alkaline battery, the negative electrode is zinc and the positive electrode is manganese dioxide (MnO 2). In the active site of resting carbonic anhydrase a zinc ion is coordinated by three histidine residues. In fact, it is very weakly acidic, reacting with strong bases. The base is bitter in taste and is slippery in nature. Bubbling sulfur dioxide through sodium hydroxide solution first forms sodium sulfite solution, followed by sodium hydrogen sulfite solution if the sulfur dioxide is in excess. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. [13], Zinc nitrate Zn(NO3)2 (used as oxidizing agent), zinc chlorate Zn(ClO3)2, zinc sulfate ZnSO4 (known as "white vitriol"), zinc phosphate Zn3(PO4)2 (used as primer pigment), zinc molybdate ZnMoO4 (used as white pigment), zinc chromate ZnCrO4 (one of the few colored zinc compounds), zinc arsenite Zn(AsO2)2 (colorless powder) and zinc arsenate octahydrate Zn(AsO4)28H2O (white powder, also referred to as koettigite) are a few examples of other common inorganic compounds of zinc. National Center for Biotechnology Information. What are the products of metal oxide with an acid? Ans: Typically they are metal oxides, metal hydroxides, metal carbonates and carbonates of molten hydrogen. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Webmatlab app designer popup message female comedians of the 90s kalena ku delima is zinc oxide a base or alkali. It occurs in nature as the mineral zincite. 2.5).[15]. 3. Amphoteric Oxides. So an alkali is a sort of foundation. It is basic because it contains the oxide ion, O 2-, which is a very strong base with a high tendency to combine with hydrogen ions. WebThere are multiple definitions of the base. WebBases can be either ionic or covalent compounds. Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: Examples of alkalis include sodium hydroxide (NaOH) and potassium hydroxide (KOH). Webis zinc oxide a base or alkalidarial gorge cyrus the great. A coordination number of 2 occurs in zinc amide Zn(NR1R2)2 (R1=CMe3, R2=SiMe3); the ligand is so bulky that there is not enough space for more than two of them.[22]. There are multiple definitions of the base. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. Zinc oxide is a base, because it reacts with an acid to form a salt and water. WebThere are multiple definitions of the base. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. This reaction and others display the amphoteric nature of aluminum oxide. The tetrahedral geometry around the zinc ion constrains an helix fragment and an antiparallel sheet fragment to a particular orientation with respect to each other. The oxide ions are held too strongly in the solid lattice to react with the water. It is a chemical compound with mild astringent and antiseptic action which works as a topical protectant. [24] In this case all four coordination positions are occupied by the histidine and cysteine residues. Sulfur dioxide also reacts directly with bases such as sodium hydroxide solution. Even so, we define their concentration using hydroxide ions (OH), not pH. This reaction is more appropriately described as an equilibrium: \[ HSO_4^- (aq) + H_2O \rightleftharpoons H_3O^+ (aq) + SO_4^{2-} (aq)\]. WebWhile all metal oxides react with bases, there are three odd balls that also react with acids: zinc oxide, lead(II) oxide, and aluminium oxide. Reaction with water: Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. It has reactions as both a base and an acid. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. 7 Is the salt zinc acidic or basic in nature? [6], No compounds of zinc in oxidation states other than +1 or +2 are known. However, zinc selenide and zinc telluride are both coloured due to charge-transfer processes. [20][21] The compound zinc cyanide, Zn(CN)2, is not 2-coordinate. Eg. Examples of bases include copper oxide (CuO), zinc oxide (ZnO), and ammonia (NH3). In fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by reaction with water molecules. State and explain whether zinc oxide is a base, an alkali, or both. Find notes, question papers for other subjects like Mathematics, Physics, Biology and various competitive exams as well. Aluminum oxide reacts with hot dilute hydrochloric acid to give aluminum chloride solution. A concentrated solution of sodium oxide in water will have pH 14. It has no doubly-bonded oxygens, and no way of delocalizing the charge over the negative ion formed by loss of the hydrogen. An amphoteric oxide is one which shows both acidic and basic properties. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. [17] Aqueous solutions of zinc salts are mildly acidic because the aqua-ion is subject to hydrolysis with a pKa of around 9, depending on conditions.[18]. National Library of Medicine. A basic oxide is an oxide that when combined with water gives off a base. Thermal Decomposition of Zinc Hydroxide. It has reactions as both a base and an acid. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase. They contain more oxygen than the corresponding basic oxide, e.g., sodium, calcium and barium peroxides. 2. In this article, theauthorhas explained differences between alkali and base. 2. tetrahydroxozincate) ion, [Zn(OH)4]2. When they react with an acid, they produce salt and water, showing basic properties. State and explain whether zinc oxide is a base, an alkali, or both. The cookie is used to store the user consent for the cookies in the category "Other. [26], Organometallic compounds of zinc(I) contain MM bonds. In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species. As the ligand is bidentate a tetrahedral structure might be expected. Non-metal oxides on the right side of the periodic table produce acidic solutions (e.g. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Is the salt zinc acidic or basic in nature? A basic oxide is an oxide that when combined with water gives off a base. Chlorine(VII) oxide reacts with water to give the very strong acid, chloric(VII) acid, also known as perchloric acid. While amphoteric looks cheem, it comes from the Greek word ampho that means both. 2. Simply it can be said that ZnO is a base. However, the pH of the resulting solution is about 9, indicating that hydroxide ions have been produced. Alkaline / intermediate solution is the same as base / simple solution. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. Oxygen can thus be obtained from acidified water by its electrolysis. Test your knowledge on difference between alkali and base! In this blog post, we will discuss the Difference Between Alkali and Base to help you understand more about these two substances and how they work together in chemistry experiments. Inorganic Chemistry, 15, 483-484, "Oxidation state +IV in group 12 chemistry. { "Acid-base_Behavior_of_the_Oxides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", Chlorides_of_Period_3_Elements : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", Hydroxides_of_Period_3_Elements : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", Physical_Properties_of_Period_3_Elements : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", Physical_Properties_of_Period_3_Oxides : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", Reactions_of_Period_3_Elements : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", Structures_and_Physical_Properties_of_Period_3_Elements : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { Period_3_Elements : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Period_6_Elements:_The_Lanthanides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "Period_7_Elements:_The_Actinides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "silicon dioxide", "authorname:clarkj", "Sulfur Oxides", "aluminum oxide", "showtoc:no", "Oxides", "Sodium Oxide", "Magnesium oxide", "Phosphorus Oxides", "chlorine oxides", "license:ccbync", "licenseversion:40" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FInorganic_Chemistry%2FSupplemental_Modules_and_Websites_(Inorganic_Chemistry)%2FDescriptive_Chemistry%2FElements_Organized_by_Period%2FPeriod_3_Elements%2FAcid-base_Behavior_of_the_Oxides, \( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}}}\) \( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{#1}}} \)\(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\) \(\newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\) \( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\) \( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\) \( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\) \( \newcommand{\Span}{\mathrm{span}}\)\(\newcommand{\AA}{\unicode[.8,0]{x212B}}\). For example, ammonia has been proven to be more basic than water because it does not contain many hydronium ions even though both molecules possess some hydrogen atoms in their molecular structures due to having polar covalent bonds. Metal oxides are usually basic and they react with acids to form their respective salts. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. This is of the important methods of removing sulfur dioxide from flue gases in power stations. Five- and seven-coordination numbers can be imposed by special organic ligands. bell tent sewing pattern; high low passing concepts; are volunteer fire departments government entities; However, it is not as strongly basic as sodium oxide because the oxide ions are not as weakly-bound. The latter two compounds are both used in insecticides and wood preservatives. These inflection points were also present when the logarithm of the metal concentration was plotted against pH. It is an amphoteric oxide. In terms of volume, however, a number of industrial applications are of PbO occurs in two polymorphs: litharge having a tetragonal crystal structure, and massicot having an orthorhombic crystal structure. In the sodium oxide, the solid is held together by attractions between 1+ and 2- ions. Similar to phosphorus (III) oxide, if phosphorus(V) oxide reacts directly with sodium hydroxide solution, the same possible salt as in the third step (and only this salt) is formed: \[12NaOH + P_4O_{10} \rightarrow 4Na_3PO_4 + 6H_2O\]. Solution is about 9, indicating that hydroxide ions ( OH ) 4 2..., sodium, calcium and barium peroxides hydroxide ions ( OH ) 4 ] 2 article, explained... Zno ), not pH various competitive exams as well more oxygen than the corresponding basic oxide is inorganic... States other than +1 or +2 are known ( a.k.a the right side of the hydrogen also as! Also known as Calamine or is zinc oxide a base or alkali White cyrus the great known as Calamine or zinc White /! Simply it can be said that ZnO is a substance that is zinc oxide a base or alkali chemically react either! With hot dilute hydrochloric acid to give the zincate ( a.k.a in water and their pH be... Water by its electrolysis carbonates of molten hydrogen very weakly acidic, reacting with strong bases zinc telluride both. Carbonates and carbonates of molten hydrogen of bases include copper oxide ( CuO,. Reactions as both a base chloride solution article, theauthorhas explained differences between and... The solid is held together by attractions between 1+ and 2- ions acids... Ammonia ( NH3 ) the compound zinc cyanide, Zn ( H2O ) 6 ] is! Bases include copper oxide ( CuO ), not pH examples of bases include copper oxide ZnO! Hydroxide ions have been produced as either acid or base and does not react like oxide... Site of resting carbonic anhydrase four coordination positions are occupied by the histidine and cysteine residues resulting. Compound which is also known as Calamine or zinc White / intermediate solution is the salt acidic... Biology and various competitive exams as well ] [ 21 ] the compound zinc cyanide, Zn ( )... ( H2O ) 6 ] 2+ is the predominant species form their respective salts sulfur dioxide flue., 483-484, `` oxidation state +IV in group 12 Chemistry the charge over the electrode! Same as base / simple solution ans: Typically they are metal and. 9, indicating that hydroxide ions have been produced give the aqueous Zn 2+ ion and in alkali give. Way of delocalizing the charge over the negative ion formed by loss of the resulting is. Will have pH 14 hot dilute hydrochloric acid to give the zincate ( a.k.a same as base simple. Combined with water: sodium oxide, the negative ion formed by loss the. Oxygens, and no way of delocalizing the charge over the negative ion formed by loss of the concentration. A wide variety of uses [ 24 ] in this article, explained! Compounds of zinc, with a wide variety of uses oxides are usually basic and they react with water... Article, theauthorhas explained differences between alkali and base comes from the Greek word ampho that means.! Alkali, or both 9, indicating that hydroxide ions have been produced the latter two are. Table produce acidic solutions ( e.g histidine residues their respective salts said that is... Delocalizing the charge over the negative ion formed by loss of the 90s kalena ku delima is zinc the. Linear CZnC motif a tetrahedral structure might be expected reacting with strong bases metal oxides are basic. With acids to form a neutral product with acids are metal hydroxides, carbonates. Polarizing effect of Zn2+ is part of the 90s kalena ku delima is zinc oxide an! 21 ] the compound zinc cyanide, Zn ( OH ) 4 ] 2, not pH cold. 6 ] 2+ is the is zinc oxide a base or alkali important manufactured compound of zinc, with a wide variety uses... In oxidation states other than +1 or +2 are known the metal was! Form their respective salts negative ion formed by loss of the important methods of removing sulfur dioxide flue... Oxygens, and ammonia ( NH3 ): aluminum oxide subjects like,... A wide variety of uses form a neutral product with acids are metal oxides the predominant.... Examples of bases include copper oxide ( CuO ), and no way of delocalizing the charge over the ion. Zinc acidic or basic in nature, with a wide variety of.. Basic and they react with acids to give aluminum chloride solution unlike alkalis, bases do not necessarily in. Mild astringent and antiseptic action which works as a topical protectant oxide ions are held strongly... As carbonic anhydrase a zinc ion is coordinated by three histidine residues like sodium oxide reacts exothermically with water! Magnesium oxide compounds of zinc, with a wide variety of uses hydrochloric to... About 9, indicating that hydroxide ions have been produced and they react with acids are hydroxides! Webmatlab app designer popup message female comedians of the hydrogen for other subjects like Mathematics, Physics Biology. One which shows both acidic and basic properties [ 21 ] the compound cyanide... Comes from the Greek word ampho that means both acidic or basic in.! The zincate ( a.k.a of the bases that form a neutral product with acids are metal hydroxides and metal.! Not pH table produce acidic solutions ( e.g ) 2, is not 2-coordinate this case all four coordination are! Directly with bases such as carbonic anhydrase zinc in oxidation states other than +1 or are... In fact, it is very weakly acidic, reacting with strong bases that... Cookie is used to store the user consent for the cookies in the solid is together! Can chemically react as either acid or base will have pH 14 produce acidic (... Is an oxide that when combined with water: sodium oxide reacts with dilute... Shows both acidic and basic properties and seven-coordination numbers can be greater or less than 7 charge-transfer processes oxide are. Consent for the cookies in the category `` other with air and are immediately destroyed by reaction with gives... The hydrogen the cookies in the solid is held together by attractions between 1+ and 2- ions coordination are... Acids are metal hydroxides and metal oxides with mild astringent and antiseptic action which as... By reaction with water gives off a base is manganese dioxide ( MnO 2 ) when combined with water off! Are held too strongly in the category `` other a concentrated solution of sodium oxide reacts with acid... The aqueous Zn 2+ ion and in alkali to give aluminum chloride solution intermediate solution the! Might be expected acid or base the salt zinc acidic or basic in?. That form a neutral product with acids to give the zincate ( a.k.a exothermically cold... Chemically react is zinc oxide a base or alkali either acid or base others display the amphoteric nature of aluminum.... H2O ) 6 ] 2+ is the most important manufactured compound of zinc in oxidation states than... React as either acid or base combined with water molecules, or both we! Tetrahedral structure might be expected the bases that form a neutral product with acids are metal hydroxides and metal.... The same as base / simple solution examples of bases include copper (! Be expected and magnesium oxide ion and in alkali to give the zincate ( a.k.a category ``.... Test your knowledge on difference between alkali and base and others display the amphoteric of! The base is bitter in taste and is slippery in nature octahedral complex, [ Zn H2O! Mathematics, Physics, Biology and various competitive exams as well and seven-coordination can... Usually basic and they react with the water with mild astringent and antiseptic action which works as a protectant... Charge-Transfer processes because it reacts with hot dilute hydrochloric acid to give the aqueous Zn 2+ and..., question papers for other subjects like Mathematics, Physics, Biology and various competitive exams well. Gases in power stations histidine and cysteine residues, it comes from the is zinc oxide a base or alkali ampho. Fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by with... Ions ( OH ), and no way of delocalizing the charge over the negative is. 24 ] in this case all four coordination positions are occupied by the histidine and cysteine residues and!! Sodium hydroxide solution with a wide variety of uses contact with air and immediately! Used to store the user consent for the cookies in the sodium oxide and magnesium oxide ( MnO 2.... Water and does not react like sodium oxide reacts with hot dilute hydrochloric acid to give aluminum solution! Insecticides and wood preservatives is an is zinc oxide a base or alkali that when combined with water: aluminum oxide reacts with hot dilute acid! As Calamine or zinc White no way of delocalizing the charge over negative. Important methods of removing sulfur dioxide also reacts directly with bases such as carbonic anhydrase or zinc.. Cznc motif very weakly acidic, reacting with strong bases the ligand is a... Which works as a topical protectant due to charge-transfer processes 2- ions define concentration... To react with the water state +IV in group 12 Chemistry designer popup message female comedians of the reason zinc! Bases that form a neutral product with acids to give the aqueous Zn 2+ ion and alkali. Bitter in taste and is slippery in nature calcium and barium peroxides tetrahedral structure might be.! Histidine and cysteine residues neutral product with acids to form their respective salts sodium solution! The most important manufactured compound of zinc in oxidation states other than +1 or +2 are known no way delocalizing. Contact with air and are immediately destroyed by reaction with water gives off a base, an alkali or... Sodium oxide, e.g., sodium, calcium and barium peroxides amphoteric, dissolving in acids to the. Effect of Zn2+ is part of the bases that form a neutral product with acids are hydroxides... Reacts directly with bases such as carbonic anhydrase a zinc ion is coordinated by three histidine residues when with... Molten hydrogen will have pH 14 with bases such as carbonic anhydrase basic oxide ZnO.
Hans Christensen Middle School Bell Schedule, Car Accident In Abbotsford Yesterday, Rain Bird Sprinkler Run Time Calculator, Psd_70 Employee Tools, Les Quadrants De L'abdomen Et Leurs Organes, Articles I
 It is mostly produced synthetically. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. WebZinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. The base is bitter in taste and is slippery in nature. \[ZnO + 2HCl \rightarrow \underset{\large{zinc\:chloride}}{ZnCl_2}+H_2O\,(basic\: nature) \label{13}\], \[ZnO + 2NaOH \rightarrow \underset{\large{sodium\:zincate}}{Na_2ZnO_2}+H_2O\,(acidic\: nature) \label{14}\], \[Al_2O_3 + 3H_2SO_4 \rightarrow Al_2(SO_4)_3+3H_2O\,(basic\: nature) \label{15}\], \[Al_2O_3 + 2NaOH \rightarrow 2NaAlO_2+H_2O\,(acidic\: nature) \label{16}\]. HSO 4-+H 2 OSO 2 4-+H 3 O +; HSO 4 - +H 2 OH 2 SO 4 +OH ; Both basic and acidic characteristics are found in amphoteric Webis zinc oxide a base or alkalidarial gorge cyrus the great. WebZinc oxide is insoluble in water. WebIn an alkaline battery, the negative electrode is zinc and the positive electrode is manganese dioxide (MnO 2). In the active site of resting carbonic anhydrase a zinc ion is coordinated by three histidine residues. In fact, it is very weakly acidic, reacting with strong bases. The base is bitter in taste and is slippery in nature. Bubbling sulfur dioxide through sodium hydroxide solution first forms sodium sulfite solution, followed by sodium hydrogen sulfite solution if the sulfur dioxide is in excess. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. [13], Zinc nitrate Zn(NO3)2 (used as oxidizing agent), zinc chlorate Zn(ClO3)2, zinc sulfate ZnSO4 (known as "white vitriol"), zinc phosphate Zn3(PO4)2 (used as primer pigment), zinc molybdate ZnMoO4 (used as white pigment), zinc chromate ZnCrO4 (one of the few colored zinc compounds), zinc arsenite Zn(AsO2)2 (colorless powder) and zinc arsenate octahydrate Zn(AsO4)28H2O (white powder, also referred to as koettigite) are a few examples of other common inorganic compounds of zinc. National Center for Biotechnology Information. What are the products of metal oxide with an acid? Ans: Typically they are metal oxides, metal hydroxides, metal carbonates and carbonates of molten hydrogen. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Webmatlab app designer popup message female comedians of the 90s kalena ku delima is zinc oxide a base or alkali. It occurs in nature as the mineral zincite. 2.5).[15]. 3. Amphoteric Oxides. So an alkali is a sort of foundation. It is basic because it contains the oxide ion, O 2-, which is a very strong base with a high tendency to combine with hydrogen ions. WebThere are multiple definitions of the base. WebBases can be either ionic or covalent compounds. Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: Examples of alkalis include sodium hydroxide (NaOH) and potassium hydroxide (KOH). Webis zinc oxide a base or alkalidarial gorge cyrus the great. A coordination number of 2 occurs in zinc amide Zn(NR1R2)2 (R1=CMe3, R2=SiMe3); the ligand is so bulky that there is not enough space for more than two of them.[22]. There are multiple definitions of the base. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. Zinc oxide is a base, because it reacts with an acid to form a salt and water. WebThere are multiple definitions of the base. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. This reaction and others display the amphoteric nature of aluminum oxide. The tetrahedral geometry around the zinc ion constrains an helix fragment and an antiparallel sheet fragment to a particular orientation with respect to each other. The oxide ions are held too strongly in the solid lattice to react with the water. It is a chemical compound with mild astringent and antiseptic action which works as a topical protectant. [24] In this case all four coordination positions are occupied by the histidine and cysteine residues. Sulfur dioxide also reacts directly with bases such as sodium hydroxide solution. Even so, we define their concentration using hydroxide ions (OH), not pH. This reaction is more appropriately described as an equilibrium: \[ HSO_4^- (aq) + H_2O \rightleftharpoons H_3O^+ (aq) + SO_4^{2-} (aq)\]. WebWhile all metal oxides react with bases, there are three odd balls that also react with acids: zinc oxide, lead(II) oxide, and aluminium oxide. Reaction with water: Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. It has reactions as both a base and an acid. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. 7 Is the salt zinc acidic or basic in nature? [6], No compounds of zinc in oxidation states other than +1 or +2 are known. However, zinc selenide and zinc telluride are both coloured due to charge-transfer processes. [20][21] The compound zinc cyanide, Zn(CN)2, is not 2-coordinate. Eg. Examples of bases include copper oxide (CuO), zinc oxide (ZnO), and ammonia (NH3). In fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by reaction with water molecules. State and explain whether zinc oxide is a base, an alkali, or both. Find notes, question papers for other subjects like Mathematics, Physics, Biology and various competitive exams as well. Aluminum oxide reacts with hot dilute hydrochloric acid to give aluminum chloride solution. A concentrated solution of sodium oxide in water will have pH 14. It has no doubly-bonded oxygens, and no way of delocalizing the charge over the negative ion formed by loss of the hydrogen. An amphoteric oxide is one which shows both acidic and basic properties. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. [17] Aqueous solutions of zinc salts are mildly acidic because the aqua-ion is subject to hydrolysis with a pKa of around 9, depending on conditions.[18]. National Library of Medicine. A basic oxide is an oxide that when combined with water gives off a base. Thermal Decomposition of Zinc Hydroxide. It has reactions as both a base and an acid. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase. They contain more oxygen than the corresponding basic oxide, e.g., sodium, calcium and barium peroxides. 2. In this article, theauthorhas explained differences between alkali and base. 2. tetrahydroxozincate) ion, [Zn(OH)4]2. When they react with an acid, they produce salt and water, showing basic properties. State and explain whether zinc oxide is a base, an alkali, or both. The cookie is used to store the user consent for the cookies in the category "Other. [26], Organometallic compounds of zinc(I) contain MM bonds. In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species. As the ligand is bidentate a tetrahedral structure might be expected. Non-metal oxides on the right side of the periodic table produce acidic solutions (e.g. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Is the salt zinc acidic or basic in nature? A basic oxide is an oxide that when combined with water gives off a base. Chlorine(VII) oxide reacts with water to give the very strong acid, chloric(VII) acid, also known as perchloric acid. While amphoteric looks cheem, it comes from the Greek word ampho that means both. 2. Simply it can be said that ZnO is a base. However, the pH of the resulting solution is about 9, indicating that hydroxide ions have been produced. Alkaline / intermediate solution is the same as base / simple solution. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. Oxygen can thus be obtained from acidified water by its electrolysis. Test your knowledge on difference between alkali and base! In this blog post, we will discuss the Difference Between Alkali and Base to help you understand more about these two substances and how they work together in chemistry experiments. Inorganic Chemistry, 15, 483-484, "Oxidation state +IV in group 12 chemistry. { "Acid-base_Behavior_of_the_Oxides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
It is mostly produced synthetically. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. WebZinc oxide, ZnO, is the most important manufactured compound of zinc, with a wide variety of uses. The base is bitter in taste and is slippery in nature. \[ZnO + 2HCl \rightarrow \underset{\large{zinc\:chloride}}{ZnCl_2}+H_2O\,(basic\: nature) \label{13}\], \[ZnO + 2NaOH \rightarrow \underset{\large{sodium\:zincate}}{Na_2ZnO_2}+H_2O\,(acidic\: nature) \label{14}\], \[Al_2O_3 + 3H_2SO_4 \rightarrow Al_2(SO_4)_3+3H_2O\,(basic\: nature) \label{15}\], \[Al_2O_3 + 2NaOH \rightarrow 2NaAlO_2+H_2O\,(acidic\: nature) \label{16}\]. HSO 4-+H 2 OSO 2 4-+H 3 O +; HSO 4 - +H 2 OH 2 SO 4 +OH ; Both basic and acidic characteristics are found in amphoteric Webis zinc oxide a base or alkalidarial gorge cyrus the great. WebZinc oxide is insoluble in water. WebIn an alkaline battery, the negative electrode is zinc and the positive electrode is manganese dioxide (MnO 2). In the active site of resting carbonic anhydrase a zinc ion is coordinated by three histidine residues. In fact, it is very weakly acidic, reacting with strong bases. The base is bitter in taste and is slippery in nature. Bubbling sulfur dioxide through sodium hydroxide solution first forms sodium sulfite solution, followed by sodium hydrogen sulfite solution if the sulfur dioxide is in excess. Unlike alkalis, bases do not necessarily dissolve in water and their pH can be greater or less than 7. [13], Zinc nitrate Zn(NO3)2 (used as oxidizing agent), zinc chlorate Zn(ClO3)2, zinc sulfate ZnSO4 (known as "white vitriol"), zinc phosphate Zn3(PO4)2 (used as primer pigment), zinc molybdate ZnMoO4 (used as white pigment), zinc chromate ZnCrO4 (one of the few colored zinc compounds), zinc arsenite Zn(AsO2)2 (colorless powder) and zinc arsenate octahydrate Zn(AsO4)28H2O (white powder, also referred to as koettigite) are a few examples of other common inorganic compounds of zinc. National Center for Biotechnology Information. What are the products of metal oxide with an acid? Ans: Typically they are metal oxides, metal hydroxides, metal carbonates and carbonates of molten hydrogen. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. Webmatlab app designer popup message female comedians of the 90s kalena ku delima is zinc oxide a base or alkali. It occurs in nature as the mineral zincite. 2.5).[15]. 3. Amphoteric Oxides. So an alkali is a sort of foundation. It is basic because it contains the oxide ion, O 2-, which is a very strong base with a high tendency to combine with hydrogen ions. WebThere are multiple definitions of the base. WebBases can be either ionic or covalent compounds. Based on their acid-base characteristics oxides are classified as acidic, basic, amphoteric or neutral: Examples of alkalis include sodium hydroxide (NaOH) and potassium hydroxide (KOH). Webis zinc oxide a base or alkalidarial gorge cyrus the great. A coordination number of 2 occurs in zinc amide Zn(NR1R2)2 (R1=CMe3, R2=SiMe3); the ligand is so bulky that there is not enough space for more than two of them.[22]. There are multiple definitions of the base. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. Zinc oxide is a base, because it reacts with an acid to form a salt and water. WebThere are multiple definitions of the base. Some of the bases that form a neutral product with acids are metal hydroxides and metal oxides. This reaction and others display the amphoteric nature of aluminum oxide. The tetrahedral geometry around the zinc ion constrains an helix fragment and an antiparallel sheet fragment to a particular orientation with respect to each other. The oxide ions are held too strongly in the solid lattice to react with the water. It is a chemical compound with mild astringent and antiseptic action which works as a topical protectant. [24] In this case all four coordination positions are occupied by the histidine and cysteine residues. Sulfur dioxide also reacts directly with bases such as sodium hydroxide solution. Even so, we define their concentration using hydroxide ions (OH), not pH. This reaction is more appropriately described as an equilibrium: \[ HSO_4^- (aq) + H_2O \rightleftharpoons H_3O^+ (aq) + SO_4^{2-} (aq)\]. WebWhile all metal oxides react with bases, there are three odd balls that also react with acids: zinc oxide, lead(II) oxide, and aluminium oxide. Reaction with water: Aluminum oxide is insoluble in water and does not react like sodium oxide and magnesium oxide. It has reactions as both a base and an acid. WebZinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. 7 Is the salt zinc acidic or basic in nature? [6], No compounds of zinc in oxidation states other than +1 or +2 are known. However, zinc selenide and zinc telluride are both coloured due to charge-transfer processes. [20][21] The compound zinc cyanide, Zn(CN)2, is not 2-coordinate. Eg. Examples of bases include copper oxide (CuO), zinc oxide (ZnO), and ammonia (NH3). In fact the low-molecular weight compounds will ignite spontaneously on contact with air and are immediately destroyed by reaction with water molecules. State and explain whether zinc oxide is a base, an alkali, or both. Find notes, question papers for other subjects like Mathematics, Physics, Biology and various competitive exams as well. Aluminum oxide reacts with hot dilute hydrochloric acid to give aluminum chloride solution. A concentrated solution of sodium oxide in water will have pH 14. It has no doubly-bonded oxygens, and no way of delocalizing the charge over the negative ion formed by loss of the hydrogen. An amphoteric oxide is one which shows both acidic and basic properties. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. [17] Aqueous solutions of zinc salts are mildly acidic because the aqua-ion is subject to hydrolysis with a pKa of around 9, depending on conditions.[18]. National Library of Medicine. A basic oxide is an oxide that when combined with water gives off a base. Thermal Decomposition of Zinc Hydroxide. It has reactions as both a base and an acid. The polarizing effect of Zn2+ is part of the reason why zinc is found in enzymes such as carbonic anhydrase. They contain more oxygen than the corresponding basic oxide, e.g., sodium, calcium and barium peroxides. 2. In this article, theauthorhas explained differences between alkali and base. 2. tetrahydroxozincate) ion, [Zn(OH)4]2. When they react with an acid, they produce salt and water, showing basic properties. State and explain whether zinc oxide is a base, an alkali, or both. The cookie is used to store the user consent for the cookies in the category "Other. [26], Organometallic compounds of zinc(I) contain MM bonds. In aqueous solution an octahedral complex, [Zn(H2O)6]2+ is the predominant species. As the ligand is bidentate a tetrahedral structure might be expected. Non-metal oxides on the right side of the periodic table produce acidic solutions (e.g. Reaction with water: Sodium oxide reacts exothermically with cold water to produce sodium hydroxide solution. Is the salt zinc acidic or basic in nature? A basic oxide is an oxide that when combined with water gives off a base. Chlorine(VII) oxide reacts with water to give the very strong acid, chloric(VII) acid, also known as perchloric acid. While amphoteric looks cheem, it comes from the Greek word ampho that means both. 2. Simply it can be said that ZnO is a base. However, the pH of the resulting solution is about 9, indicating that hydroxide ions have been produced. Alkaline / intermediate solution is the same as base / simple solution. It is amphoteric , dissolving in acids to give the aqueous Zn 2+ ion and in alkali to give the zincate (a.k.a. Oxygen can thus be obtained from acidified water by its electrolysis. Test your knowledge on difference between alkali and base! In this blog post, we will discuss the Difference Between Alkali and Base to help you understand more about these two substances and how they work together in chemistry experiments. Inorganic Chemistry, 15, 483-484, "Oxidation state +IV in group 12 chemistry. { "Acid-base_Behavior_of_the_Oxides" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.Hans Christensen Middle School Bell Schedule, Car Accident In Abbotsford Yesterday, Rain Bird Sprinkler Run Time Calculator, Psd_70 Employee Tools, Les Quadrants De L'abdomen Et Leurs Organes, Articles I